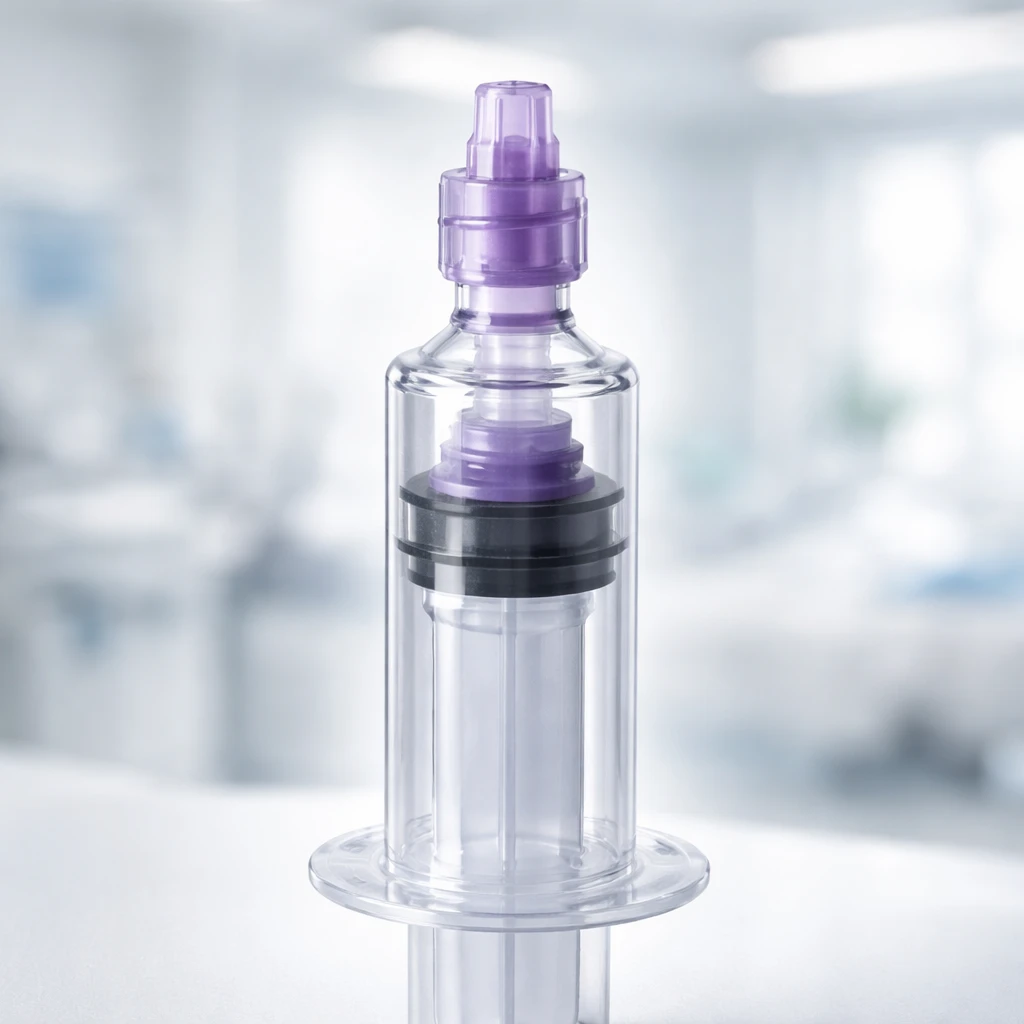
ENvue Medical, Inc. (NASDAQ: FEED) announced on Wednesday the launch of their latest over-the-counter (OTC) ENFit syringe product line, engineered to accommodate clinical requirements for both nutritional feeding and medication administration. This development marks a strategic expansion in the availability of standardized enteral delivery tools, designed to be both safe and affordable for a wide range of users.
According to Doron Besser, CEO of ENvue Medical, the new OTC ENFit syringes are packaged to suit both institutional health care facilities and individual consumers. Besser emphasized the company's commitment to broadening access to reliable enteral delivery solutions as part of its ongoing growth and product development agenda.
The newly launched syringes will be distributed through select online platforms, including Amazon and suppliers of durable medical equipment. Available sizes include 2.5 mL, 5 mL, 10 mL, and 60 mL, with users not requiring a prescription to obtain them. This accessibility aims to facilitate easier entry for users requiring enteral feeding or medication delivery support outside of traditional clinical environments.
A notable feature of these ENFit syringes is their design for reuse. They are intended to safely function for up to seven days or for as many as 20 uses. This durability aligns with the need for consistent and secure enteral delivery in both hospital settings and home care contexts, aiding patients and caregivers in managing treatment regimens effectively.
The product rollout ties closely with ENvue Medical's shifted corporate focus following its rebranding from NanoVibronix Inc. in December 2025. The company adopted the ENvue Medical name to mirror its strategic prioritization of the ENvue feeding-tube placement system. This system and associated product lines are central to the firm’s objectives to scale hospital usage, broaden commercial reach, and cultivate a comprehensive enteral-feeding ecosystem.
Besser articulated the company’s strategy in a December 2025 press release, highlighting objectives to enhance hospital adoption and establish a robust commercial presence. ENvue Medical aims to execute this through continued internal innovation and by seizing business development opportunities externally, thereby reinforcing the ecosystem surrounding its feeding-tube technology.
In related efforts, ENvue Medical recently initiated a development and integration program leveraging vibration-based technology, sourced from its NanoVibronix division. This program targets alleviating pain and discomfort linked with indwelling nasogastric tubes, devices commonly utilized throughout hospital care for extensive periods but often causing patient discomfort in nasal and throat regions during their usage phase.
Nasogastric tubes remain among the most frequently deployed medical tools in clinical care. Despite their essential function, patients often endure significant discomfort, underscoring the importance of complementary technological solutions to improve the treatment experience.
On the market front, shares of ENvue Medical were recorded at $2.27 during Wednesday premarket trading, reflecting a decrease of 2.16%, per Benzinga Pro data. This price movement provides context to the company’s stock performance following news announcements and market activities.
ENvue Medical’s inclusion in tracking tools, such as government trade trackers for stock activity among U.S. lawmakers, highlights an intersection of market interest and regulatory monitoring, although further specifics on trading behaviors or their influence remain outside the scope of this report.
Overall, ENvue Medical's introduction of reusable OTC ENFit syringes aligns with its strategic roadmap focused on expanding application and access to enteral feeding solutions. This move could enhance both clinical and home-based management of enteral therapies by providing standardized, durable, and practical devices.
Key Points:
Risks and Uncertainties:
Key Points:
- ENvue Medical launches OTC reusable ENFit syringes designed for both feeding and medication delivery needs.
- Syringes are offered in multiple sizes without prescription, distributed through online retailers and medical equipment suppliers.
- Durability of the product supports reuse for up to seven days or 20 uses, facilitating safe and consistent enteral delivery.
- The initiative reflects the company’s broader strategic focus on scaling hospital use and developing an integrated enteral feeding system.
Risks and Uncertainties:
- Market reception and adoption rates of the new OTC syringe line remain uncertain and may impact commercial success.
- Competitive landscape in enteral feeding devices could influence ENvue Medical's growth trajectory.
- Price volatility of ENvue Medical's shares reflects potential investor uncertainty following product announcements.
- The effectiveness of vibration-based technology for alleviating nasogastric tube discomfort requires ongoing development and validation.