The Food and Drug Administration (FDA) has expanded the availability of novel diagnostic and treatment methods targeting some of the most prevalent sexually transmitted infections (STIs) in the United States, a move anticipated to contribute to lowering national infection rates.
In recent developments finalized last year, the FDA authorized several at-home testing products. Among these is the first over-the-counter test designed specifically to identify three common infections among women: gonorrhea, chlamydia, and trichomoniasis. Alongside this, the FDA approved the first home sampling kit intended for detecting human papillomavirus (HPV), the primary virus linked to cervical cancer.
In addition to testing advancements, the FDA approved two new oral treatments for gonorrhea—the first approvals in decades for this resilient bacterial infection—providing alternatives to the current injectable antibiotic.
These approvals arrive as public health officials manage declining STI rates following several years marked by disrupted services amid the COVID-19 pandemic that affected screening, education, and treatment nationwide.
The pandemic period also catalyzed progress in diagnostic technology. Techniques originally developed for over-the-counter COVID-19 testing platforms have translated into new at-home solutions for infections such as syphilis and other STIs, which were previously mostly limited to clinical settings.
Dr. Ina Park, a sexual health expert at the University of California, San Francisco, noted that stigma and reluctance often impede individuals from seeking sexual health services in traditional clinical environments. The increased accessibility of home testing kits provides an alternative route that may encourage more people to get tested.
Convenience and Speed in Testing
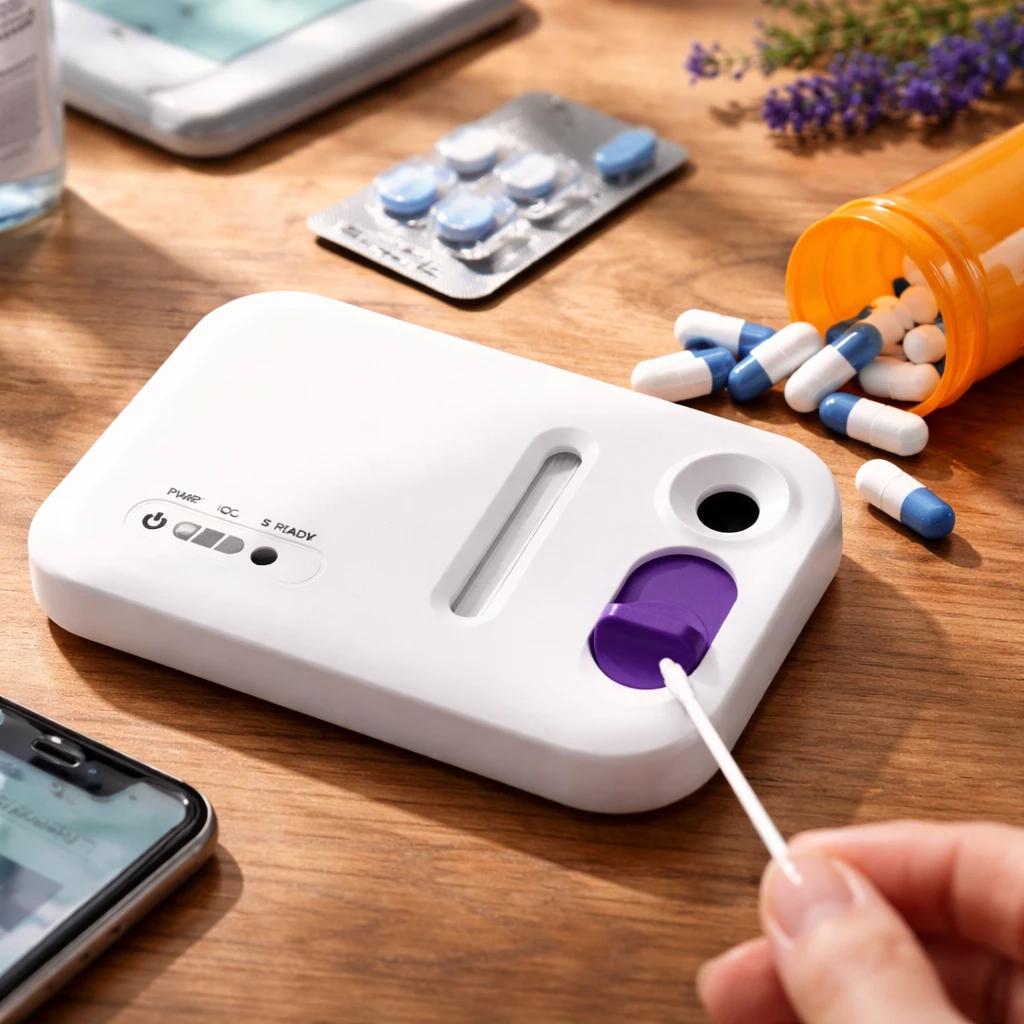
One such home test on the market is Visby Medical's three-in-one diagnostic kit, FDA-approved in March of the previous year. This test employs a urine sample and a vaginal swab, using a small electronic device that analyzes the sample and delivers results to a connected smartphone application.
Priced at $150, the package also includes a telehealth consultation with a licensed medical provider, who can interpret results and prescribe treatment remotely if infections are detected. This streamlined approach reduces the entire process—from purchase to prescription—to about six hours, a considerable improvement compared to the multi-day timeframe typical in traditional clinical testing and result delivery, explained Dr. Gary Schoolnik, Visby's chief medical officer and professor emeritus at Stanford Medical School.
Previously, testing usually involved sample collection by healthcare personnel, lab-based analysis, and subsequent in-person follow-ups, which frequently resulted in delays or loss to follow-up for positive cases.
The FDA's approval of Visby's device was based on clinical studies demonstrating detection accuracies of approximately 98% or higher for the three targeted infections, comparable to conventional clinical testing.
Other home-based testing technologies necessitate external laboratory processing. For example, the FDA recently approved Teal Health's HPV testing kit, which enables women to collect vaginal samples themselves and mail them to a lab for analysis. This coincides with updated federal screening guidelines endorsing self-collected samples for HPV testing.
New Treatments Address Antibiotic Resistance
Addressing treatment difficulties, the FDA authorized the first new oral gonorrhea medications in decades. Gonorrhea-causing bacteria have developed resistance to almost all previously used antibiotics.
The new treatments include Nuzolvence, administered as granules dissolved in water, developed through a public-private partnership, and Bluejeja, an oral tablet from GlaxoSmithKline also approved for urinary tract infections. Both drugs can be taken orally, avoiding the need for the current standard injectable antibiotic ceftriaxone.
Previous CDC guidelines recommended combining injectable ceftriaxone with oral azithromycin; however, azithromycin was removed from guidance due to increasing resistance. Dr. Ina Park highlighted the significance of having two new options available in the same year after a prolonged period of limited treatments.
Infection Trends and Emerging Challenges
According to the Centers for Disease Control and Prevention's (CDC) preliminary data for 2024, rates for gonorrhea have decreased for the third consecutive year, with additional declines in adult chlamydia and infectious syphilis cases observed over recent years.
Experts attribute these trends to multiple factors, including changes in sexual behaviors, broader use of antibiotics to prevent infections, and enhanced home screening availability.
However, increased home testing raises concerns about public health surveillance accuracy, as traditionally, data reporting has relied on large laboratory networks. Shifts in testing settings could complicate monitoring of national infection rates.
Cost barriers also pose challenges; for example, the Visby test’s $150 price is typically not covered by insurance, potentially limiting widespread adoption. Compounding this, recent reductions in funding for the CDC and other public health agencies under the prior federal administration may hamper access to sexual health services, especially for underserved populations.
Dr. Park expressed optimism about the expanded testing options and new drugs but voiced concerns that funding cuts might restrict access to these advances for those least able to afford them.
These developments represent a significant junction for sexual health care delivery in the United States, reflecting technological advancements and evolving treatment landscapes while underscoring the continuing need for equitable access and robust public health infrastructure.