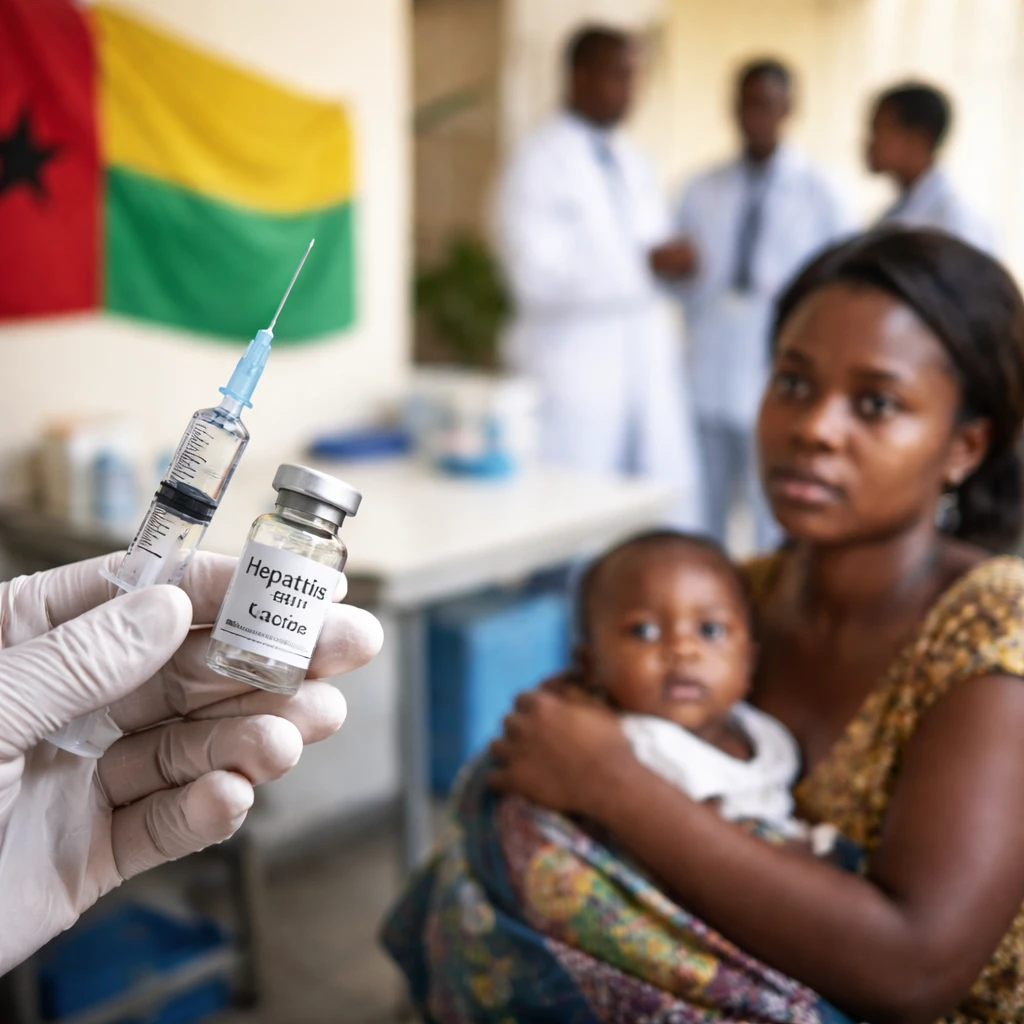
In a recent development, the West African nation of Guinea-Bissau has decided to pause a hepatitis B vaccination trial targeting newborn infants. The pause, confirmed by Health Minister Quinhi Nantot, will allow for a comprehensive ethical review after concerns were raised about the study’s design and review process.
The trial, initially endorsed by the U.S. government under the previous administration, is structured as a randomized controlled study. It involves administering the hepatitis B vaccine to some newborns at birth while withholding it from others, with ongoing follow-up to track mortality rates, disease incidence, and developmental outcomes over time. The methodology has attracted criticism from various health experts who perceive the withholding of the vaccine from some children—especially in a region with high hepatitis B prevalence—as ethically questionable.
During a press conference held by the Africa Centers for Disease Control and Prevention (Africa CDC), Minister Nantot revealed that the local six-member ethics committee had not convened to formally review and approve the study when initially confirmed. This procedural irregularity prompted calls for reassessment to ensure the protection of participants’ wellbeing and adherence to ethical standards.
Jean Kaseya, Director-General of Africa CDC, publicly endorsed the decision to carry out a thorough ethical review. He emphasized the agency’s commitment to prioritizing the health interests of African citizens over narrower individual agendas, underscoring the strong collaborative relationship between Africa CDC and the U.S. government in public health initiatives.
Despite Guinea-Bissau’s suspension, U.S. health authorities have communicated that the study is poised to proceed as originally planned. In a recent statement, Andrew Nixon, spokesperson for the U.S. Department of Health and Human Services, affirmed ongoing collaboration with partners to finalize the clinical protocols.
This vaccine study originated from a $1.6 million no-bid contract awarded by the former U.S. administration to a university in Denmark. The researchers attached to the project have drawn scrutiny due to affiliations cited by vaccine skeptics and questions raised by prominent health experts regarding their scientific rigor. The prevailing medical consensus supports the vaccine’s protective benefits for newborns, rendering the study's approach of withholding it from a subset of infants a matter of ethical concern.
The United States Centers for Disease Control and Prevention funded a research team at the University of Southern Denmark to carry out this investigation. The team has been recognized by U.S. Health Secretary Robert F. Kennedy Jr., notable for his controversial stance on vaccine policies. One leading researcher, Christine Stabell Benn, serves as a consultant for a committee appointed by Kennedy, which recently opted against recommending the hepatitis B vaccine routinely for all newborns in the United States.
Planned to commence earlier in the year, the research is slated to enroll 14,000 newborns over a five-year period in Guinea-Bissau, a country grappling with significant rates of hepatitis B infection. Most participants are to be monitored for less than two years to evaluate vaccine side effects, while the first 500 infants recruited will undergo extended observation for five years to assess potential impacts on behavior and neurological development.
As initially conceived, the trial did not include a placebo group. Details of any modifications to the study design have not been publicly disclosed by U.S. officials. Such adjustments could be part of ongoing discussions linked to the ethical review and stakeholder consultations.
The unfolding situation highlights the complexities inherent in conducting vaccine research in vulnerable populations, particularly when ethical standards and local oversight mechanisms are brought into question. Stakeholders on both sides emphasize the importance of safeguarding participant welfare while advancing public health knowledge.